News

Sliding Into Winter 2025!

By PCCD Watershed Specialist Rachael Marques
It’s that time of year again! The leaves have fallen, and temperatures are dropping. Soon we will be seeing the first snowflakes of the winter season. Along with the snow comes ice!
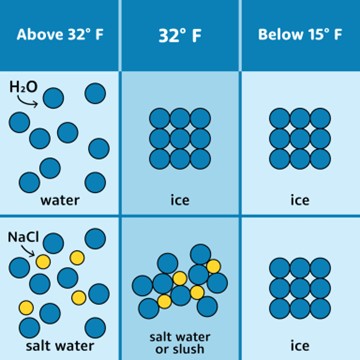
There are several different ways to approach icy weather in our area. The most used tool has many nicknames: road salt, ice melt, rock salt, etc. These terms actually encompass a few different chemicals. The most common are sodium chloride, magnesium chloride, and calcium chloride, all of which are salts! These salts mix with the wintry precipitation to lower the freezing point of water on the road (see graphic to right). This prevents water on the road from freezing into icy patches and instead keeps it slushy which is less slick. Slush is also much easier to remove or push away meaning safer long-term conditions.
This salt doesn’t just disappear after its job is done though, it is absorbed into the ground and nearby waterways. Salty water can runoff into our streams creating salty conditions for sensitive wildlife. Things such as fish, plants, and other stream critters in our area are adapted to freshwater conditions. Extra-salty environments can cause stress in an ecosystem and can even be toxic to the flora and fauna within it. Salty water can also percolate into our aquifers, which would add salt to our fresh drinking water sources. This can in turn cause infrastructure damage, plumbing issues, and can even make your water taste salty!
Needless to say, we want to avoid the conditions in the paragraph above, but how? We certainly are not calling for stopping the use of road salts. These salts are critical for maintaining save travel conditions for people both on foot and on our roadways! Human health and safety are always a priority.
So what can we do as individuals to help prevent salinization of our resources while still keeping people safe? The first thing we can do is use road salt but use it responsibly. Always read the instructions on any chemical you purchase, including ice melt. Many of us, myself included, were led to believe that you need to feel the “crunch” of the rock salt on sidewalks and pavement to know it is working. This is not true! According to the Izaak Walton League, “one of America’s oldest and most successful conservation organizations”: in general, for a 20 ft driveway all you need is one 12oz mug of salt to prevent ice formation!
Another common misconception is that salt eliminates the need for shoveling and plowing. These are still required to prevent unsafe winter conditions. The Izaak Walton League has a great motto for the winter months: Shovel, Scatter, Sweep, Repeat. Step one is to shovel snow away before it can turn into ice, you can even sweep or blow light snow away. The next step is to scatter! Scatter the ice melt with the consideration a 12oz mug is enough for almost two full parking spaces. The third step, sweep, is important as well. Did you know you can sweep up leftover or spilled ice melt and re-use it for the next storm? This can save money and our freshwater!
For environmentally friendly alternatives for smaller areas around the home, visit this article from Penn State Extension.
If you are interested in learning more about salt use and the effects on our streams, visit the Izaak Walton League Salt Watch page. If you are interested in participating in their Salt Watch program, reach out to the Izaak Walton League or the Pike County Conservation District Watershed Specialist, Rachael Marques by phone (570-226-8220) or email (rmarques@pikepa.org).
Sources:
Scientific American “Salt Doesn’t Melt Ice – Here’s How It Makes Winter Streets Safer
Izaak Walton League of America – Salt Watch